Single-cell analysis and sorting using droplet-based microfluidics
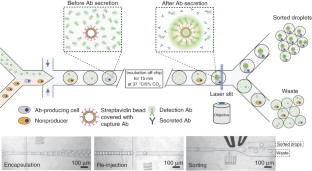
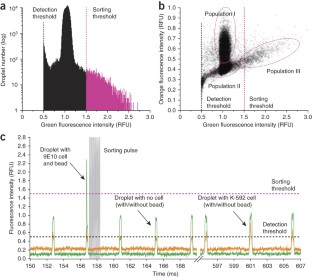
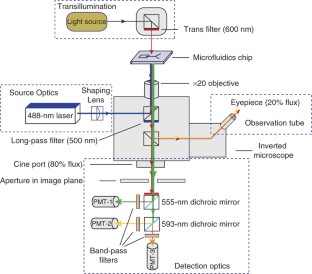
We present a droplet-based microfluidics protocol for high-throughput analysis and sorting of single cells. Compartmentalization of single cells in droplets enables the analysis of proteins released from or secreted by cells, thereby overcoming one of the major limitations of traditional flow cytometry and fluorescence-activated cell sorting. As an example of this approach, we detail a binding assay for detecting antibodies secreted from single mouse hybridoma cells. Secreted antibodies are detected after only 15 min by co-compartmentalizing single mouse hybridoma cells, a fluorescent probe and single beads coated with anti-mouse IgG antibodies in 50-pl droplets. The beads capture the secreted antibodies and, when the captured antibodies bind to the probe, the fluorescence becomes localized on the beads, generating a clearly distinguishable fluorescence signal that enables droplet sorting at ∼ 200 Hz as well as cell enrichment. The microfluidic system described is easily adapted for screening other intracellular, cell-surface or secreted proteins and for quantifying catalytic or regulatory activities. In order to screen ∼ 1 million cells, the microfluidic operations require 2–6 h; the entire process, including preparation of microfluidic devices and mammalian cells, requires 5–7 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Design and construction of a microfluidics workstation for high-throughput multi-wavelength fluorescence and transmittance activated droplet analysis and sorting
Article 27 January 2023
Image-Based Single Cell Sorting Automation in Droplet Microfluidics
Article Open access 26 May 2020
Dynamic single-cell phenotyping of immune cells using the microfluidic platform DropMap
Article 12 August 2020
References
- Guo, M.T., Rotem, A., Heyman, J.A. & Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip12, 2146–2155 (2012). ArticleCASPubMedGoogle Scholar
- Kintses, B., van Vliet, L.D., Devenish, S.R. & Hollfelder, F. Microfluidic droplets: new integrated workflows for biological experiments. Curr. Opin. Chem. Biol.14, 548–555 (2010). ArticleCASPubMedGoogle Scholar
- Theberge, A.B. et al. Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed.49, 5846–5868 (2010). ArticleCASGoogle Scholar
- Dove, A. Drug screening--beyond the bottleneck. Nat. Biotechnol.17, 859–863 (1999). ArticleCASPubMedGoogle Scholar
- Link, D.R., Anna, S.L., Weitz, D.A. & Stone, H.A. Geometrically mediated breakup of drops in microfluidic devices. Phys. Rev. Lett.92, 054503 (2004). ArticleCASPubMedGoogle Scholar
- Mazutis, L., Baret, J.C. & Griffiths, A.D. A fast and efficient microfluidic system for highly selective one-to-one droplet fusion. Lab Chip9, 2665–2672 (2009). ArticleCASPubMedGoogle Scholar
- Mazutis, L. & Griffiths, A.D. Selective droplet coalescence using microfluidic systems. Lab Chip12, 1800–1806 (2012). ArticleCASPubMedGoogle Scholar
- Ahn, K., Agresti, J., Chong, H., Marquez, M. & Weitz, D.A. Electrocoalescence of drops synchronized by size-dependent flow in microfluidic channels. Appl. Phys. Lett.88, 264105–264103 (2006). ArticleCASGoogle Scholar
- Chabert, M., Dorfman, K.D. & Viovy, J.L. Droplet fusion by alternating current (AC) field electrocoalescence in microchannels. Electrophoresis26, 3706–3715 (2005). ArticleCASPubMedGoogle Scholar
- Priest, C., Herminghaus, S. & Seemann, R. Controlled electrocoalescence in microfluidics: targeting a single lamella. Appl. Phys. Lett.89, 134101 (2006). ArticleCASGoogle Scholar
- Abate, A.R., Hung, T., Mary, P., Agresti, J.J. & Weitz, D.A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl. Acad. Sci. USA107, 19163–19166 (2010). ArticlePubMedPubMed CentralGoogle Scholar
- Li, L. et al. Nanoliter microfluidic hybrid method for simultaneous screening and optimization validated with crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA103, 19243–19248 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Niu, X., Gulati, S., Edel, J.B. & deMello, A.J. Pillar-induced droplet merging in microfluidic circuits. Lab Chip8, 1837–1841 (2008). ArticleCASPubMedGoogle Scholar
- Frenz, L., Blank, K., Brouzes, E. & Griffiths, A.D. Reliable microfluidic on-chip incubation of droplets in delay-lines. Lab Chip9, 1344–1348 (2009). ArticleCASPubMedGoogle Scholar
- Hatch, A.C. et al. 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip11, 3838–3845 (2011). ArticleCASPubMedGoogle Scholar
- Shim, J.U. et al. Simultaneous determination of gene expression and enzymatic activity in individual bacterial cells in microdroplet compartments. J. Am. Chem. Soc.42, 15251–15256 (2009). ArticleCASGoogle Scholar
- Mazutis, L. et al. Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis. Anal. Chem.81, 4813–4821 (2009). ArticleCASPubMedGoogle Scholar
- Lichtman, J.W. & Conchello, J.A. Fluorescence microscopy. Nat. Methods2, 910–919 (2005). ArticleCASPubMedGoogle Scholar
- Najah, M., Griffiths, A.D. & Ryckelynck, M. Teaching single-cell digital analysis using droplet-based microfluidics. Anal. Chem.84, 1202–1209 (2012). ArticleCASPubMedGoogle Scholar
- Ahn, K. et al. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett.88, 024104 (2006). ArticleCASGoogle Scholar
- Franke, T., Abate, A.R., Weitz, D.A. & Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip9, 2625–2627 (2009). ArticleCASPubMedGoogle Scholar
- Debs, B.E., Utharala, R., Balyasnikova, I.V., Griffiths, A.D. & Merten, C.A. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA109, 11570–11575 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Granieri, L., Baret, J.C., Griffiths, A.D. & Merten, C.A. High-throughput screening of enzymes by retroviral display using droplet-based microfluidics. Chem. Biol.17, 229–235 (2010). ArticleCASPubMedGoogle Scholar
- He, M. et al. Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets. Anal. Chem.77, 1539–1544 (2005). ArticleCASPubMedGoogle Scholar
- Clausell-Tormos, J. et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol.15, 427–437 (2008). ArticleCASPubMedGoogle Scholar
- Koster, S. et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip8, 1110–1115 (2008). ArticleCASPubMedGoogle Scholar
- Brouzes, E. et al. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. USA106, 14195–14200 (2009). ArticlePubMedPubMed CentralGoogle Scholar
- Liu, W., Kim, H.J., Lucchetta, E.M., Du, W. & Ismagilov, R.F. Isolation, incubation, and parallel functional testing and identification by FISH of rare microbial single-copy cells from multi-species mixtures using the combination of chemistrode and stochastic confinement. Lab Chip9, 2153–2162 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hufnagel, H. et al. An integrated cell culture lab on a chip: modular microdevices for cultivation of mammalian cells and delivery into microfluidic microdroplets. Lab Chip9, 1576–1582 (2009). ArticleCASPubMedGoogle Scholar
- Zeng, Y., Novak, R., Shuga, J., Smith, M.T. & Mathies, R.A. High-performance single cell genetic analysis using microfluidic emulsion generator arrays. Anal. Chem.82, 3183–3190 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Rane, T.D., Zec, H.C., Puleo, C., Lee, A.P. & Wang, T.H. Droplet microfluidics for amplification-free genetic detection of single cells. Lab Chip12, 3341–3347 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Huebner, A. et al. Development of quantitative cell-based enzyme assays in microdroplets. Anal. Chem.80, 3890–3896 (2008). ArticleCASPubMedGoogle Scholar
- Baret, J.C., Beck, Y., Billas-Massobrio, I., Moras, D. & Griffiths, A.D. Quantitative cell-based reporter gene assays using droplet-based microfluidics. Chem. Biol.17, 528–536 (2010). ArticleCASPubMedGoogle Scholar
- Huebner, A. et al. Quantitative detection of protein expression in single cells using droplet microfluidics. Chem. Commun. (Camb)28, 1218–1220 (2007). ArticleCASGoogle Scholar
- Chen, D. et al. The chemistrode: a droplet-based microfluidic device for stimulation and recording with high temporal, spatial, and chemical resolution. Proc. Natl. Acad. Sci. USA105, 16843–16848 (2008). ArticlePubMedPubMed CentralGoogle Scholar
- Niu, X., Gielen, F., Edel, J.B. & deMello, A.J. A microdroplet dilutor for high-throughput screening. Nat. Chem.3, 437–442 (2011). ArticleCASPubMedGoogle Scholar
- Mazutis, L. et al. Multi-step microfluidic droplet processing: kinetic analysis of an in vitro–translated enzyme. Lab Chip9, 2902–2908 (2009). ArticleCASPubMedGoogle Scholar
- Pekin, D. et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip11, 2156–2166 (2011). ArticleCASPubMedGoogle Scholar
- Zhong, Q. et al. Multiplex digital PCR: breaking the one target per color barrier of quantitative PCR. Lab Chip11, 2167–2174 (2011). ArticleCASPubMedGoogle Scholar
- Hindson, B.J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem.83, 8604–8610 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tewhey, R. et al. Microdroplet-based PCR enrichment for large-scale targeted sequencing. Nat. Biotechnol.27, 1025–1031 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Miller, O.J. et al. High-resolution dose-response screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA109, 378–383 (2012). ArticlePubMedGoogle Scholar
- Clausell-Tormos, J., Griffiths, A.D. & Merten, C.A. An automated two-phase microfluidic system for kinetic analyses and the screening of compound libraries. Lab Chip10, 1302–1307 (2010). ArticleCASPubMedGoogle Scholar
- Churski, K. et al. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip12, 1629–1637 (2012). ArticleCASPubMedGoogle Scholar
- Agresti, J.J. et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA107, 4004–4009 (2010). ArticlePubMedPubMed CentralGoogle Scholar
- Kintses, B. et al. Picoliter cell lysate assays in microfluidic droplet compartments for directed enzyme evolution. Chem. Biol.19, 1001–1009 (2012). ArticleCASPubMedGoogle Scholar
- Paegel, B.M. & Joyce, G.F. Microfluidic compartmentalized directed evolution. Chem. Biol.17, 717–724 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lowe, K.C. Perfluorochemical respiratory gas carriers: benefits to cell culture systems. J. Fluorine Chem.118, 19–26 (2002). ArticleCASGoogle Scholar
- Scott, R.L. The solubility of fluorocarbons. J. Am. Chem. Soc.70, 4090–4093 (1948). ArticleCASPubMedGoogle Scholar
- Simons, J.H. & Linevsky, M.J. The solubility of organic solids in fluorocarbon derivatives. J. Am. Chem. Soc.74, 4750–4751 (1952). ArticleCASGoogle Scholar
- Tawfik, D.S. & Griffiths, A.D. Man-made cell-like compartments for molecular evolution. Nat. Biotechnol.16, 652–656 (1998). ArticleCASPubMedGoogle Scholar
- Baret, J.C. et al. Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab Chip9, 1850–1858 (2009). ArticleCASPubMedGoogle Scholar
- Martino, C. et al. Intracellular protein determination using droplet-based immunoassays. Anal. Chem.83, 5361–5368 (2011). ArticleCASPubMedGoogle Scholar
- Beer, N.R. et al. On-chip single-copy real-time reverse-transcription PCR in isolated picoliter droplets. Anal. Chem.80, 1854–1858 (2008). ArticleCASPubMedGoogle Scholar
- Schaerli, Y. et al. Continuous-flow polymerase chain reaction of single-copy DNA in microfluidic microdroplets. Anal. Chem.81, 302–306 (2009). ArticleCASPubMedGoogle Scholar
- Beer, N.R. et al. On-chip, real-time, single-copy polymerase chain reaction in picoliter droplets. Anal. Chem.79, 8471–8475 (2007). ArticleCASPubMedGoogle Scholar
- Olsen, M.J. et al. Function-based isolation of novel enzymes from a large library. Nat. Biotechnol.18, 1071–1074 (2000). ArticleCASPubMedGoogle Scholar
- Aharoni, A. et al. High-throughput screening methodology for the directed evolution of glycosyltransferases. Nat. Methods3, 609–614 (2006). ArticleCASPubMedGoogle Scholar
- Unger, M.A., Chou, H.P., Thorsen, T., Scherer, A. & Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science288, 113–116 (2000). ArticleCASPubMedGoogle Scholar
- Weinstein, J.A., Jiang, N., White, R.A. III, Fisher, D.S. & Quake, S.R. High-throughput sequencing of the zebrafish antibody repertoire. Science324, 807–810 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Fan, H.C., Wang, J., Potanina, A. & Quake, S.R. Whole-genome molecular haplotyping of single cells. Nat. Biotechnol.29, 51–57 (2011). ArticleCASPubMedGoogle Scholar
- Lecault, V. et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat. Methods8, 581–586 (2011). ArticleCASPubMedGoogle Scholar
- Love, J.C., Ronan, J.L., Grotenbreg, G.M., van der Veen, A.G. & Ploegh, H.L. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat. Biotechnol.24, 703–707 (2006). ArticleCASPubMedGoogle Scholar
- Taly, V., Pekin, D., El Abed, A. & Laurent-Puig, P. Detecting biomarkers with microdroplet technology. Trends Mol. Med.18, 405–416 (2012). ArticleCASPubMedGoogle Scholar
- Choi, J.W., Kang, D.K., Park, H., deMello, A.J. & Chang, S.I. High-throughput analysis of protein-protein interactions in picoliter-volume droplets using fluorescence polarization. Anal. Chem.84, 3849–3854 (2012). ArticleCASPubMedGoogle Scholar
- Joensson, H.N., Zhang, C., Uhlen, M. & Andersson-Svahn, H. A homogeneous assay for protein analysis in droplets by fluorescence polarization. Electrophoresis33, 436–439 (2012). ArticleCASPubMedGoogle Scholar
- Srisa-Art, M., Dyson, E.C., Demello, A.J. & Edel, J.B. Monitoring of real-time streptavidin-biotin binding kinetics using droplet microfluidics. Anal. Chem.80, 7063–7067 (2008). ArticleCASPubMedGoogle Scholar
- Cecchini, M.P. et al. Ultrafast surface enhanced resonance Raman scattering detection in droplet-based microfluidic systems. Anal. Chem.83, 3076–3081 (2011). ArticleCASPubMedGoogle Scholar
- Reymond, J.L., Fluxa, V.S. & Maillard, N. Enzyme assays. Chem. Commun.2009, 34–46 (2009). Google Scholar
- Joensson, H.N. et al. Detection and analysis of low-abundance cell-surface biomarkers using enzymatic amplification in microfluidic droplets. Angew. Chem. Int. Ed. Engl.48, 2518–2521 (2009). ArticleCASPubMedGoogle Scholar
- Xia, Y.N. & Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed.37, 551–575 (1998). ArticleGoogle Scholar
- Anna, S.L., Bontoux, N. & Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett.82, 364–366 (2003). ArticleCASGoogle Scholar
- Garstecki, P., Fuerstman, M.J., Stone, H.A. & Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip6, 437–446 (2006). ArticleCASPubMedGoogle Scholar
- Abate, A.R. et al. Impact of inlet channel geometry on microfluidic drop formation. Phys. Rev. E80, 026310 (2009). ArticleCASGoogle Scholar
- Sugiura, S., Nakajima, M., Iwamoto, S. & Seki, M. Interfacial tension driven monodispersed droplet formation from microfabricated channel array. Langmuir17, 5562–5566 (2001). ArticleCASGoogle Scholar
- Baret, J.C. Surfactants in droplet-based microfluidics. Lab Chip12, 422–433 (2012). ArticleCASPubMedGoogle Scholar
- Baret, J.C., Kleinschmidt, F., El Harrak, A. & Griffiths, A.D. Kinetic aspects of emulsion stabilization by surfactants: a microfluidic analysis. Langmuir25, 6088–6093 (2009). ArticleCASPubMedGoogle Scholar
- Roach, L.S., Song, H. & Ismagilov, R.F. Controlling nonspecific protein adsorption in a plug-based microfluidic system by controlling interfacial chemistry using fluorous-phase surfactants. Anal. Chem.77, 785–796 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Holtze, C. et al. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip8, 1632–1639 (2008). ArticleCASPubMedGoogle Scholar
- Chen, F. et al. Chemical transfection of cells in picoliter aqueous droplets in fluorocarbon oil. Anal. Chem.83, 8816–8820 (2011). ArticleCASPubMedGoogle Scholar
- Hudlicky, M. & Pavlath, A.E. Chemistry of Organic Fluorine Compounds. (American Chemical Society, 1995).
- Skhiri, Y. Dynamics of molecular transport by surfactants in emulsions. Soft Matter8, 10618–10627 (2012). ArticleCASGoogle Scholar
- Woronoff, G. et al. New generation of amino coumarin methyl sulfonate-based fluorogenic substrates for amidase assays in droplet-based microfluidic applications. Anal. Chem.83, 2852–2857 (2011). ArticleCASPubMedGoogle Scholar
- Courtois, F. et al. Controlling the retention of small molecules in emulsion microdroplets for use in cell-based assays. Anal. Chem.81, 3008–3016 (2009). ArticleCASPubMedGoogle Scholar
- Siegel, A.C. et al. Cofabrication of electromagnets and microfluidic systems in poly(dimethylsiloxane). Angew Chem. Int. Ed. Engl.45, 6877–6882 (2006). ArticleCASPubMedGoogle Scholar
- Asmolov, E.S. The inertial lift on a spherical particle in a plane Poiseuille flow at large channel Reynolds number. J. Fluid Mech.381, 63–87 (1999). ArticleCASGoogle Scholar
- Di Carlo, D., Irimia, D., Tompkins, R.G. & Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA104, 18892–18897 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kemna, E.W.M. et al. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip12, 2881–2887 (2012). ArticleCASPubMedGoogle Scholar
- Ford, T., Graham, J. & Rickwood, D. Iodixanol—a nonionic isosmotic centrifugation medium for the formation of self-generated gradients. Anal. Biochem.220, 360–366 (1994). ArticleCASPubMedGoogle Scholar
- Bruce, A.T. et al. Use of iodixanol self-generated density gradients to enrich for viable urothelial cells from nonneurogenic and neurogenic bladder tissue. Tissue Eng. Part C Methods16, 33–40 (2010). ArticleCASPubMedGoogle Scholar
- Graziani-Bowering, G.M., Graham, J.M. & Filion, L.G. A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes. J. Immunol. Methods207, 157–168 (1997). ArticleCASPubMedGoogle Scholar
- Burgoyne, F. A remote syringe for cells, beads and particle injection in microfluidic channels. http://blogs.rsc.org/chipsandtips/2009/08/20/a-remote-syringe-for-cells-beads-and-particle-injection-in-microfluidic-channels/ (20 August 2009).
- Shapiro, H.M. Practical Flow Cytometry. 4th edn. (Wiley-Liss, 2003).
- Moon, S., Ceyhan, E., Gurkan, U.A. & Demirci, U. Statistical modeling of single target cell encapsulation. PLoS ONE6, e21580 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chabert, M. & Viovy, J.L. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc. Natl. Acad. Sci. USA105, 3191–3196 (2008). ArticlePubMedPubMed CentralGoogle Scholar
Acknowledgements
We are grateful to R. Sperling for the kind gift of surfactant and D. Aubrecht for his help in developing droplet detection and sorting methods. This work was supported by the National Science Foundation (NSF) (DMR-1006546), the National Institutes of Health (NIH) (P01GM096971 and 5R01EB014703-02), the Harvard Materials Science Research and Engineering Center (DMR-0820484) and the Lithuanian Research Council (MIP-048/2012). W.L.U. acknowledges support from a Canadian National Sciences and Engineering Research Council (NSERC) Postgraduate Scholarship (PGS D). J.A.H. and J.G. were supported by NIH SBIR grant no. 1R43AI082861-01. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the NSF under award no. ECS-0335765. CNS is part of Harvard University.
Author information
Authors and Affiliations
- School of Engineering and Applied Sciences (SEAS), Harvard University, Cambridge, Massachusetts, USA Linas Mazutis, W Lloyd Ung, David A Weitz & John A Heyman
- Institute of Biotechnology, Vilnius University, Vilnius, Lithuania Linas Mazutis
- HabSel Inc. Cambridge, Massachusetts, USA John Gilbert & John A Heyman
- École Supérieure de Physique et de Chimie Industrielles de la Ville de Paris (ESPCI ParisTech), Centre National de la Recherche Scientifique (CNRS) UMR 7084, Paris, France., Andrew D Griffiths
- Linas Mazutis











